- Estimating Isotopic Mass. The relative atomic mass of a carbon-12 atom is defined as 12.00 The relative atomic mass of an atom of carbon-13 is found to be 1.08333 times the mass of a carbon-12 atom, that is 1.083 × 12 = 13.00 We can estimate the mass of any isotope of an element, its isotopic mass, using its mass number (A).
- The experimental relative abundances of (M+1) isotopic ions were evaluated in different data sets. First of all, 137 solutions of commercial compounds were analyzed by flow injection analysis in both the positive and negative ion modes.
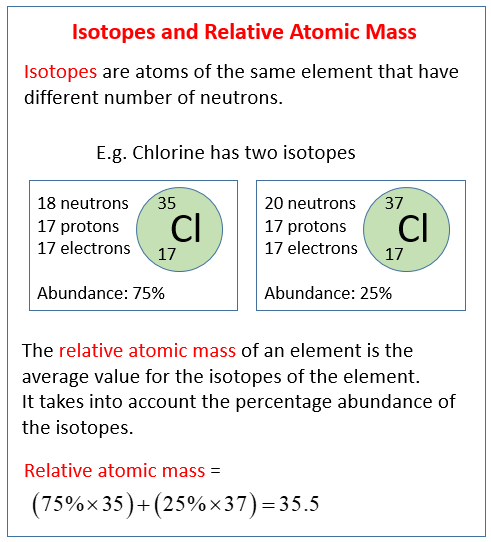
Relative atomic mass is determined by the average atomic mass, or the weighted mean of the atomic masses of all the atoms of a particular chemical element found in a particular sample, which is then compared to the atomic mass of carbon-12. This comparison is the quotient of the two weights, which makes the value dimensionless (having no unit).
1 u = 1.66 × 10-27 kg
We can estmate the the relative atomic mass (atomic weight) of an element E with the naturally occurring isotopes aE, bE, cE, etc, and with the respective abundances of A%, B %, C% etc,
%, C% etc, | relative atomic mass (r.a.m.) | = ( | A 100 | × a) | + ( | B 100 | × b) | + ( | C 100 | × c) | + etc |
and 100 - x = %abundance of isotope-b
then, let r.a.m = relative atomic mass of the element:
| r.a.m. | = ( | x 100 | × mass isotope-a) | + ( | 100 - x 100 | × mass isotope-b) |
and solve for x

Also found in: Thesaurus, Medical, Financial, Acronyms, Encyclopedia, Wikipedia.
relative atomic mass
How To Calculate Isotopic Mass
n (Chemistry) the ratio of the average mass per atom of the naturally occurring form of an element to one-twelfth the mass of an atom of carbon-12. Symbol: Ar Abbreviation: r.a.m. Former name: atomic weight
Collins English Dictionary – Complete and Unabridged, 12th Edition 2014 © HarperCollins Publishers 1991, 1994, 1998, 2000, 2003, 2006, 2007, 2009, 2011, 2014
relative atomic mass
Relative Isotopic Mass Of Cl
(atomic weight) The mass (quantity of matter) of atoms.
Dictionary of Unfamiliar Words by Diagram Group Copyright © 2008 by Diagram Visual Information Limited
Relative Isotopic Mass Meaning
| Noun | 1. | relative atomic mass - (chemistry) the mass of an atom of a chemical element expressed in atomic mass units atomic mass, atomic weight mass - the property of a body that causes it to have weight in a gravitational field combining weight, eq, equivalent weight, equivalent - the atomic weight of an element that has the same combining capacity as a given weight of another element; the standard is 8 for oxygen meq, milliequivalent - one-thousandth of an equivalent chemical science, chemistry - the science of matter; the branch of the natural sciences dealing with the composition of substances and their properties and reactions |
Based on WordNet 3.0, Farlex clipart collection. © 2003-2012 Princeton University, Farlex Inc.
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:
